Next Generation Percutaneous AV Fistula Creation
The Velocity™ Percutaneous Arterio-Venous Fistula System
About the Velocity™ pAVF System
Despite clear benefits of initiating hemodialysis with an arteriovenous fistula (AVF) including lower mortality, less reinterventions and lower healthcare costs, few patients in the U.S. do so. The Velocity pAVF System is the next generation of vascular access systems that will address patient level and system barriers for access creation while simultaneously providing quickly functioning access with less need for reintervention.
The Velocity System is designed to provide an opportunity to streamline the process to create functional dialysis access without follow up interventions.

More than 80% of patients start dialysis with a catheter.1 Catheters are a major source of morbidity and mortality for patients on hemodialysis.2 Patients outcomes are improved when dialyzing via an AVF.3

Less than 20% of patients start dialysis with an AVF: Starting dialysis with an AVF decreases the risk of infections, hospitalizations and results in lower overall healthcare costs compared to catheters. AVF use also leads to better long-term outcomes for patients.
Late diagnosis and referral delays: Many patients with chronic kidney disease do not received kidney care soon enough, leaving not enough time to create and mature an AVF before dialysis initiation.
Maturation challenges: Traditional surgical AVFs often require 12 weeks (or longer) to mature, and during this period, patients are often dependent on catheters, exposing them to additional risks. Unfortunately, many AVFs never fully mature, rendering them unsuitable for use.

Narrowing at the artery-vein junction: The most common reason for delayed or failed AVF maturation is stenosis (narrowing) at the junction of the artery and vein. This restricts blood flow, preventing the fistula from reaching the size and flow necessary for effective dialysis access.

We need a better way: Our minimally invasive solution aims to create AVFs more efficiently by overcoming common barriers to access creation and optimizing maturation which would enable patients to start dialysis safely and effectively.
References:
- United States Renal Data System. 2024 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2024.
- Wasse H. Catheter-related mortality among ESRD patients. Semin Dial. 2008;21(6):547-9. doi: 10.1111/j.1525-139X.2008.00500.x
- Al-Balas A, Shariff S, Lee T, Young C, Allon M. Clinical Outcomes and Economic Impact of Starting Hemodialysis with a Catheter after Predialysis Arteriovenous Fistula Creation. Am J Nephrol. 2019;50(3):221-227. doi: 10.1159/000502050.
Watch this video to learn more about the Velocity System
The VENOS-1 Clinical Study
The VENOS-1 study is a first-in-human clinical study conducted outside the U.S., with patients followed for one year (NCT05757726). The results demonstrate the potential of our minimally invasive pAVF procedure:
-
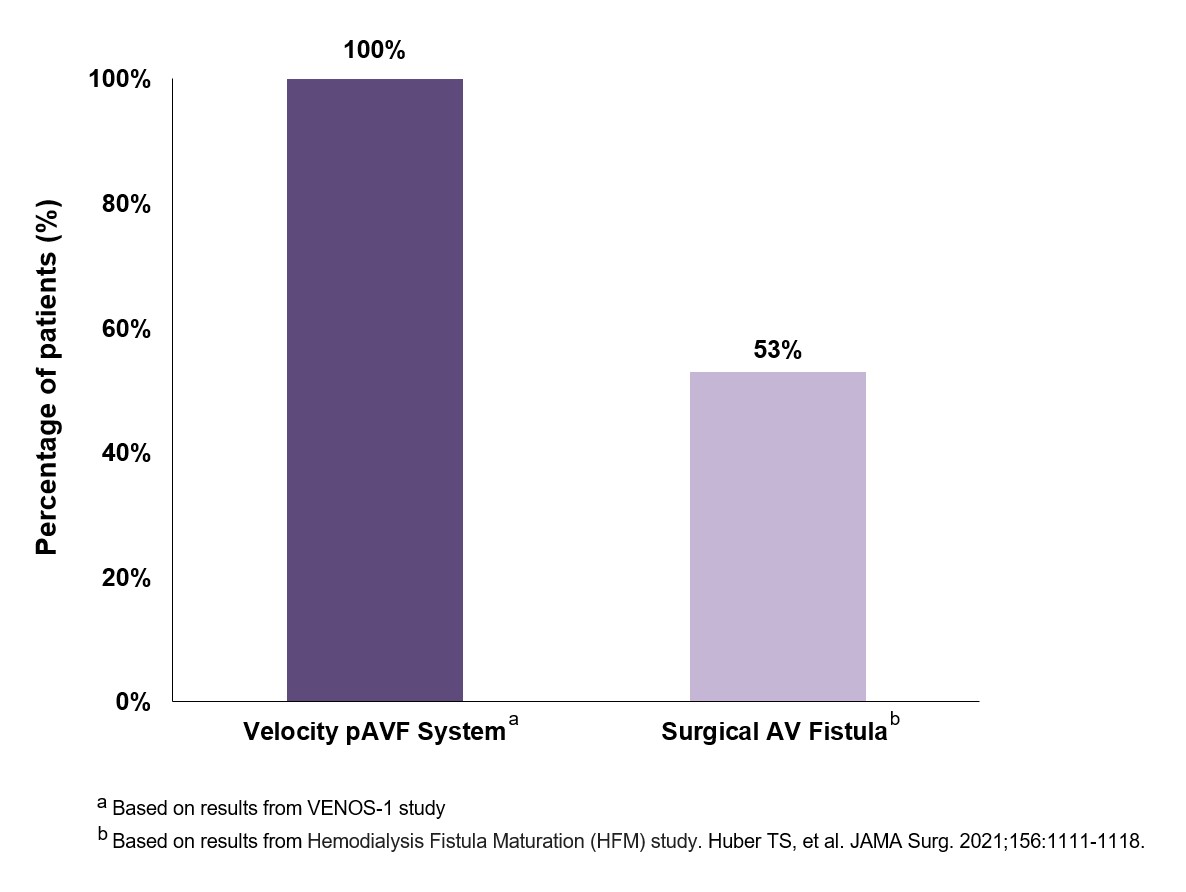
100% Technical Success, highlighting the reliability of our approach.
-
Rapid Maturation: Using a common definition of maturation—a blood flow of 500 mL/min and a vein diameter of 5 mm—all subjects met this endpoint by 6 weeks. The bar graph on the right compares physiologic fistula maturation with the Velocity System to surgical creation of an AVF.
About Us

Our Management Team
The Venova Medical team is comprised of individuals who bring a wealth of experience that covers both medical device development and clinical expertise.
Board of Directors
News and Events
Venova Medical Announces Completion of Enrollment for VENOS-2 IDE Study of the VelocityTM Percutaneous AVF System
Los Gatos, California – Venova Medical, a privately held company developing a next generation technology for the creation of percutaneous arteriovenous fistulas (pAVF) for hemodialysis access, today announced the successful completion of patient enrollment for the...
Venova Medical Announces First Subjects Enrolled in VENOS-2 IDE Study of the Velocity Percutaneous AVF System
Los Gatos, California – Venova Medical, a privately held company developing a next generation technology for the creation of percutaneous arteriovenous fistulas (pAVF) for hemodialysis access, announced today the enrollment of the first participants in the company’s...
Venova Medical to Present Results from VENOS-1 Study at American Society of Nephrology (ASN) Kidney Week 2024
(Los Gatos, California) - Venova Medical, Inc. (Venova Medical), a privately held company developing a next generation technology for the creation of percutaneous arteriovenous fistulas (pAVF) for hemodialysis access today announced that it will present an oral...